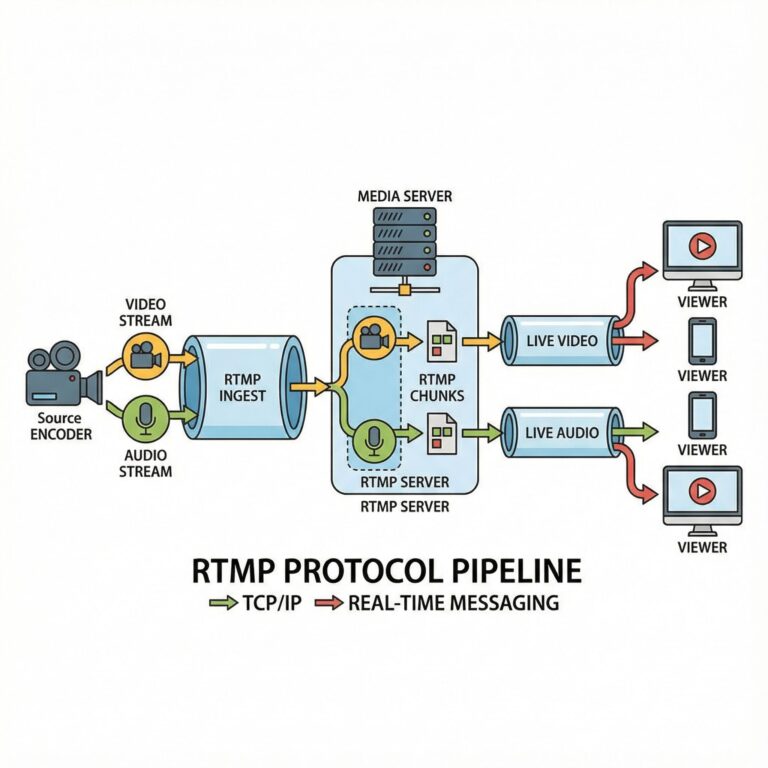
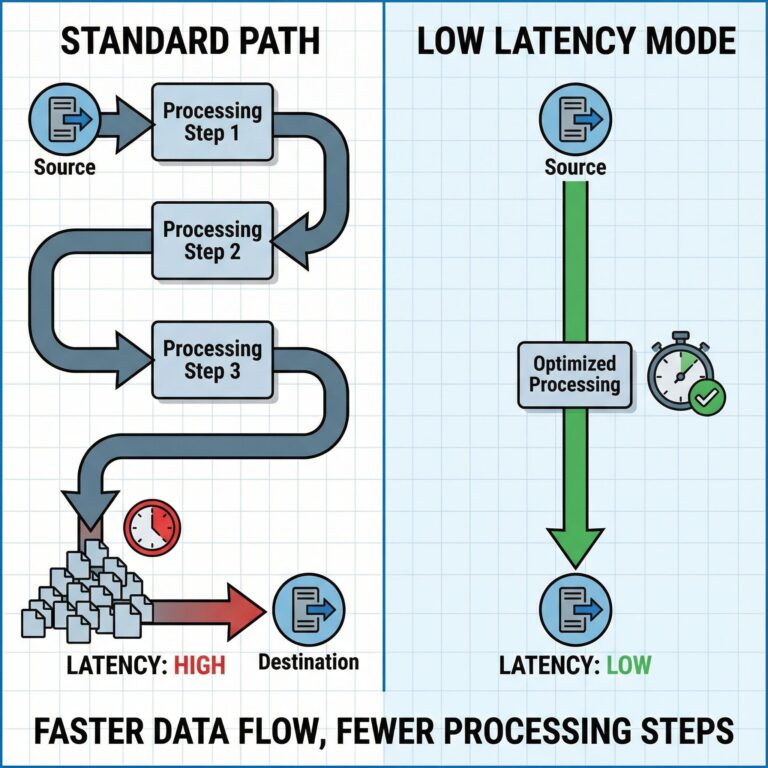
대규모 음악공연 결제 시스템의 보안 혁신과 전자음악 산업의 변화 전자음악 공연장에서의 디지털 결제 보안 현황 Otto von...
믿음 신뢰 음악가
신규 온카스터디
디지털 음악 시대의 티켓 결제 보안: 전자음악 공연장에서의 새로운 도전 전자음악 공연 티켓팅의 디지털 혁명과 보안 취약점...
Otto von Schirach의 혁신적 사운드 실험과 전자음악계의 새로운 패러다임 마이애미 언더그라운드에서 시작된 음향 혁명 전자음악의 경계를 끊임없이...
마이애미 언더그라운드에서 탄생한 충격의 아이콘 전자음악의 역사를 돌아보면 장르의 경계를 허무는 아티스트들이 존재한다. 그 중에서도 Otto von...